Hi Mike,
You ask the question, how much of the carbon you sequester into the deep sea comes from the atmosphere, and how much from the sea? The answer to this question is that initially, it all comes from the sea. However, the effect of removing the carbon is to reduce the pCO2 of the water, causing more CO2 to enter the sea from the atmosphere than before the removal, and over a period of about a year following the sequestration, the increased CO2 coming from the atmosphere will replace most (more than 90%) of the carbon you have removed. To get this result you would have to remove the carbon from a region in which there is little or no formation of subsurface water from surface water, so that the water from which you’ve removed carbon stays in the surface mixed layer and in contact therefore with the atmosphere. That should be the case for most places and seasons in the subtropics.
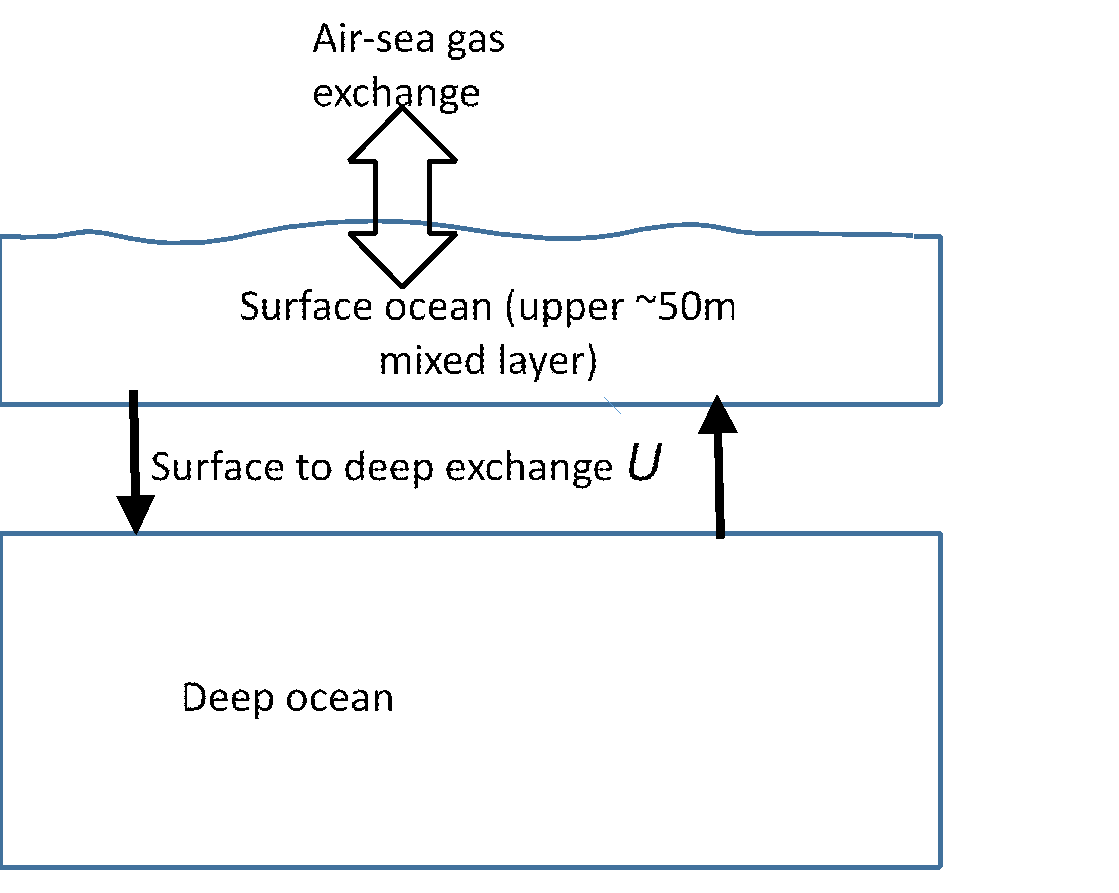
Pictured above is the simplest possible “Broecker” model of the (subtropical?) ocean, two boxes representing the surface layer above the thermocline (say top 50m) and the rest of the deep ocean. These exchange water at a rate U (cubic metres of water per year). For the whole ocean, U is something like 1015 m3yr-1, or a net ventilation rate per unit area of U/A = 3 myr-1 when averaged over the area of the global ocean A = 3.5 x 1014 m2.
The surface ocean also exchanges gases including CO2 with the atmosphere. This exchange is governed by a parameter called the air-sea gas transfer velocity, Kv which is about 1000 m yr-1. To get the flux of an “ordinary” gas that doesn’t have any complex chemistry in seawater (oxygen for example) you multiply concentration at the sea surface by the gas transfer velocity. It’s a bit more difficult for CO2 where the carbonate chemistry complicates things.
If you read the textbooks I recommended, after working through this chemistry, they give a time of one year as the time scale required for carbon in the mixed layer to equilibrate with the atmosphere. So if you cause a perturbation ΔC in the surface carbon concentration C, by for instance the growth of Sargassum which is then removed and put into the deep sea, (deep enough that it will not affect carbon coming back into the mixed layer for a long time) then over about a year this carbon will be replenished by an extra flux of CO2 in from the atmosphere. The actual time constant for this is given by
τ = C0 d / Kv α pCO20 β
where:
Kv is the gas transfer velocity, ~1000 myr-1
α is the solubility of CO2, ~35 mol m-3atm-1
pCO20 and C0 typical surface CO2 partial pressure and total carbon concentrations, e.g.
pCO20 ≈ 400 x 10-6 atm
C0 ≈ 2 molm-3
β is the Revelle buffer factor, ~10
d is the mixed layer depth, ~50m.
If I put these numbers in to the formula above, I get τ = 0.7 years, in rough agreement with the textbooks. So this is the time required for your process, which removes carbon primarily from surface seawater, to take up an almost equivalent amount of CO2 from the atmosphere.
Your potential investors might ask where else the carbon might come from to make up the deficit. Might it not come up from the deep sea instead of the atmosphere? The time scale for ventilation of the surface layer from the deep sea in the model above is dA/U = 50/3 = 16 years, again using the rough figures above, so it’s an order of magnitude slower than the ventilation via the atmosphere. Most of the deficit in carbon will therefore be made up by CO2 flowing in from the atmosphere.
(In reality, the subsurface ocean isn’t ventilated uniformly over the whole global ocean, rather it occurs episodically, dominantly in the winter and in temperate / cold waters. However you’re in good shape in the Caribbean and Gulf of Mexico and subtropics generally, the warm surface waters there are not good candidates for formation of subsurface water. What is important is that the water from which you’ve removed carbon, doesn’t get subducted below the surface for the next year or so.)